Prostate cancer treatments and
stress urinary incontinence
The most common cause of stress urinary incontinence (SUI) is prostate cancer treatment.2
Prostate cancer itself will not cause incontinence but the treatment of prostate cancer might. Treatment options for prostate cancer may affect continence by impacting the nerves or blood flow.1
After prostate cancer treatments, SUI can be temporary and improve over time, however for many men, symptoms will continue.3
After a prostatectomy,

of patients will experience moderate to severe SUI1
Approximately 21% of patients
experience both leakage and ED
after a prostatectomy1

After prostate removal,
1 in 5 men
are unhappy with their functional results likely due to incontinence and ED4
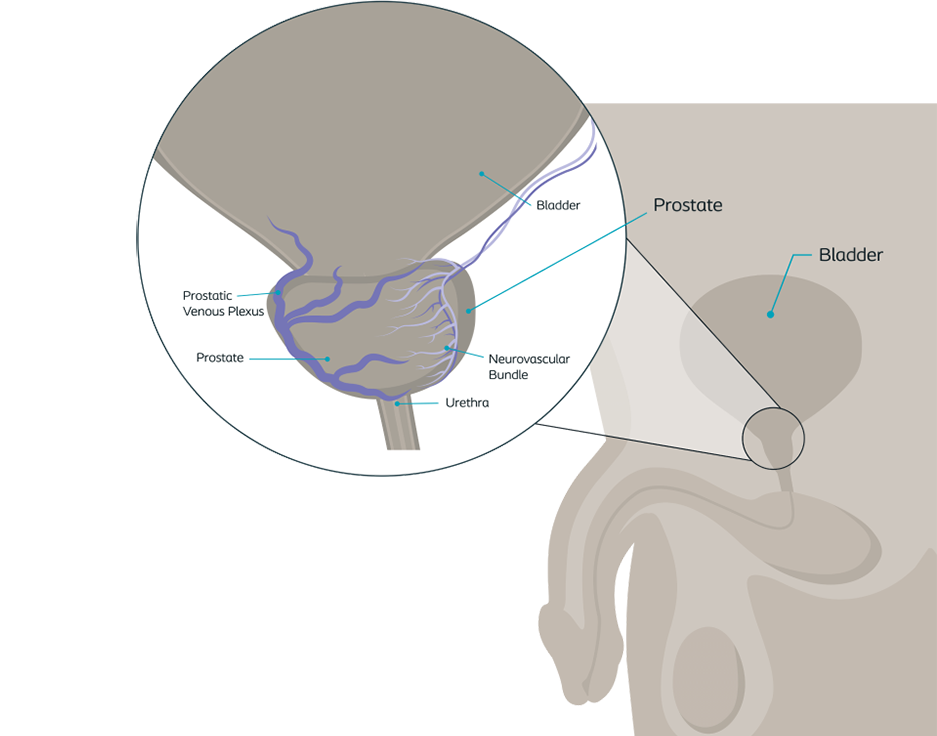
Treatment options for prostate cancer may affect continence
During a prostatectomy or radiation, damage may occur to the nerve bundles, bladder muscles, or blood vessels, which can affect continence.1
This image shows the nerves around the prostate that can be damaged during prostate removal or radiation. The prostate is around the urethra, which carries urine out of your bladder when you urinate. This can also be damaged during prostate cancer treatments. This damage can cause a weakening of the bladder muscles and less support around of the urethra, causing incontinence.
Even experienced robot-assisted surgeons completing prostate removal still experience incontinence rates of up to 10%.5
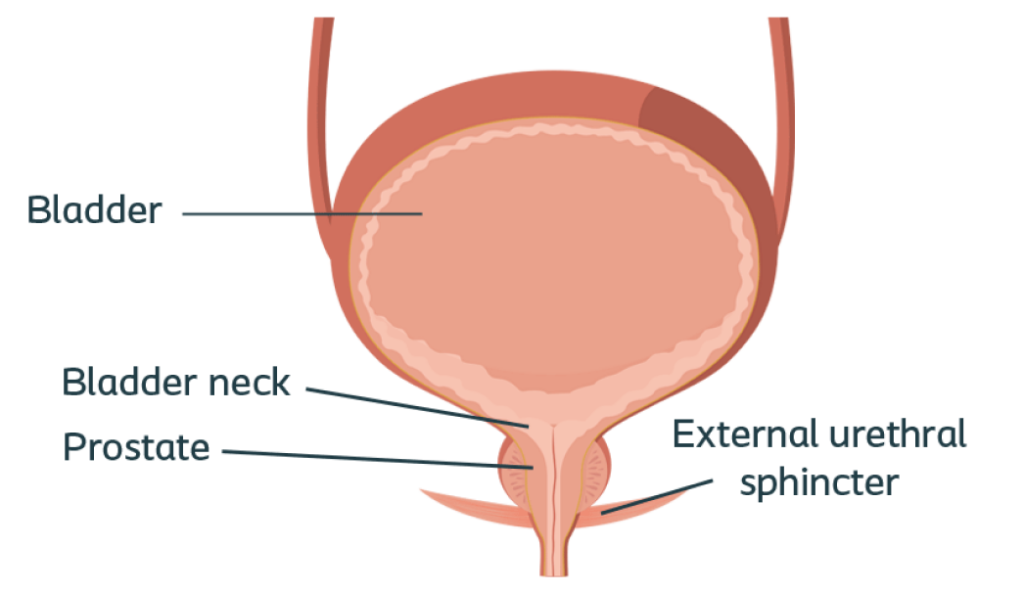
Normal prostate
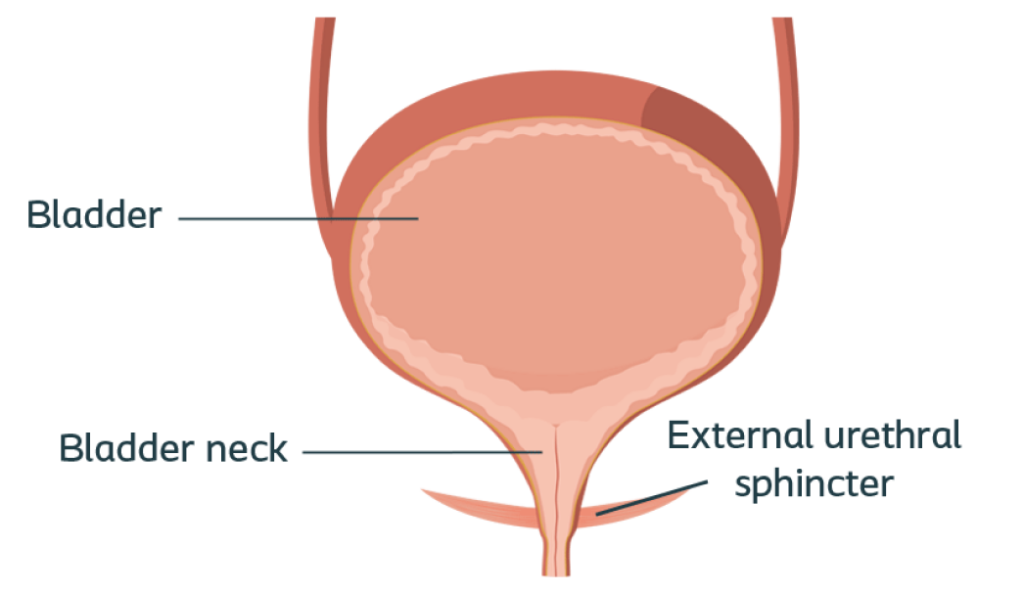
External urethral sphincter injury
post-prostatectomy
Stress urinary incontinence is treatable after prostate cancer treatments
You’ve survived prostate cancer. Now, you may be living with stress urinary incontinence as a side effect. You don’t have to live with this side effect – there are solutions available.3 Learn about the available treatment options, then speak with your physician about the right treatment option for you.
Prostate cancer and erectile dysfunction
Erectile dysfunction can also occur after prostate cancer treatments. Approximately 21% of patients experience both leakage and ED post-prostatectomy.1
If you’re facing erectile dysfunction in addition to your incontinence, it’s important to know that there are treatments available.
References
1 Tsikis ST, Nottingham CU, Faris SF. The Relationship Between Incontinence and Erectile Dysfunction After Robotic Prostatectomy: Are They Mutually Exclusive? J Sex Med. 2017 Oct;14(10):1241-1247.
2 Stress Incontinence in Men. National Association for Continence. https://nafc.org/male-stress-urinary-incontinence/. Accessed October 2023.
3 Sandhu JS, Breyer B, Comiter C, Eastham JA, Gomez C, Kirages DJ, Kittle C, Lucioni A, Nitti VW, Stoffel JT, Westney OL, Murad MH, McCammon K. Incontinence after Prostate Treatment: AUA/SUFU Guideline. J Urol. 2019 Aug;202(2):369-378.
4 Schout B, Meuleman EJ. Erectiestoornis en incontinentie na prostatectomie. Behandeling van complicaties van chirurgie bij prostaatkanker [Erectile dysfunction and incontinence after prostatectomy. Treating the complications of surgery for prostate cancer]. Ned Tijdschr Geneeskd. 2012;156(44):A4667. Dutch. PMID: 23114170.
5 Seo HJ, Lee NR, Son SK, Kim DK, Rha KH, Lee SH. Comparison of Robot-Assisted Radical Prostatectomy and Open Radical Prostatectomy Outcomes: A Systematic Review and Meta-Analysis. Yonsei Med J. 2016 Sep;57(5):1165-77.
PM-33590
Important safety information
Virtue® Male Sling System Important Safety Information
Virtue Male Sling is a polypropylene mesh device or “sling” intended to prevent involuntary urine leakage (incontinence) at times of increased pressure on the bladder (e.g., coughing, sneezing, laughing, lifting heavy objects, exercise). It is permanently implanted to support, elevate and gently compress the urethra, the tube that connects to the bladder to carry urine outside of the body.
Indications
The Virtue Male Sling System is an implantable, suburethral support sling indicated for the treatment of male stress urinary incontinence (SUI).
Contraindications
The Virtue Male Sling is contraindicated in patients with one or more of the following conditions: 1) documented hypersensitivity or allergic reaction to polypropylene, 2) active infection, including untreated urinary tract and/or infection in the operative field, 3) patients with untreated or serious blood clotting (coagulation) disorders, 4) patients with blockage of urine flow (obstructive uropathy), 5) patients under the age of 18.
Warnings
Your physician will conduct a complete evaluation with testing to confirm you are a candidate for a male sling. Patients will then be advised prior to surgery, of the warnings associated with the use of this product, the associated surgical and postoperative risks and potential complications. Sling associated complications may result in resurgery which may lead to partial or complete removal of the sling. Complete removal of the sling may not always be possible, and removal may not fully correct these complications. New onset (de novo) complications may occur. As with all surgical procedures, patients with certain underlying conditions may be more susceptible to postoperative bleeding, impaired blood supply, compromised/delayed healing, sling exposure or other complications and adverse events. The risk versus benefit of the male sling should be considered in patients with one or more of the following conditions: auto-immune disease, blood clotting (coagulation) disorder, connective tissue disease, impaired immune system (debilitated or immunocompromised state), diabetes, pelvic radiation therapy, physical characteristics (e.g., body mass index), kidney problems (renal insufficiency), smoking related conditions (e.g., COPD, chronic cough).
Potential Complications
Adverse events are known to occur with sling procedures and implants. Adverse events following sling implantation may be immediate or delayed, localized or systemic, new onset (de novo) or worsening, acute or chronic, transient or permanent.
Adverse events may include but are not limited to: allergic reaction, hypersensitivity; abnormal immune response (autoinflammatory/autoimmunity syndrome); bladder symptoms (e.g., increased daytime frequency, urgency, nocturia (urinating more than once per night), overactive bladder, urinary incontinence); bleeding/hemorrhage; delayed/impaired/abnormal wound healing; exposure, extrusion or erosion of sling into other structures or organs; fistula formation (abnormal connection or passageway between two structures in the body); foreign body granuloma (abnormal tissue formation)/scar tissue formation; genital burning / tingling / numbness (paresthesia); infection; tissue swelling/redness/discomfort (inflammation/irritation); avoidance/difficult/painful intercourse (dyspareunia / sexual dysfunction); tissue death (necrosis); weakness or loss of sensation (neuromuscular disorder); palpable mesh; pain; perforation or injury to adjacent muscles, nerves, vessels, structures, or organs (e.g., bone, bladder, urethra, ureters, bowel); collection of clear fluid or blood outside of tissue or vessels (seroma/hematoma); sling migration; urinary tract infection; inability to completely empty bladder (urinary tract obstruction); voiding symptoms (e.g., dysuria (painful urination), urinary retention, incomplete emptying, bladder outlet obstruction, straining, position-dependent voiding, slow stream).
This treatment is prescribed by your physician. Discuss the treatment options with your physician to understand the risks and benefits of the various options to determine if a male sling is right for you.
Caution: Federal law (USA) restricts this device to sale by or on the order of a physician.
PM-15544 / Apr 2024



