Torosa® Testicular Implant:
Restore part of what was lost
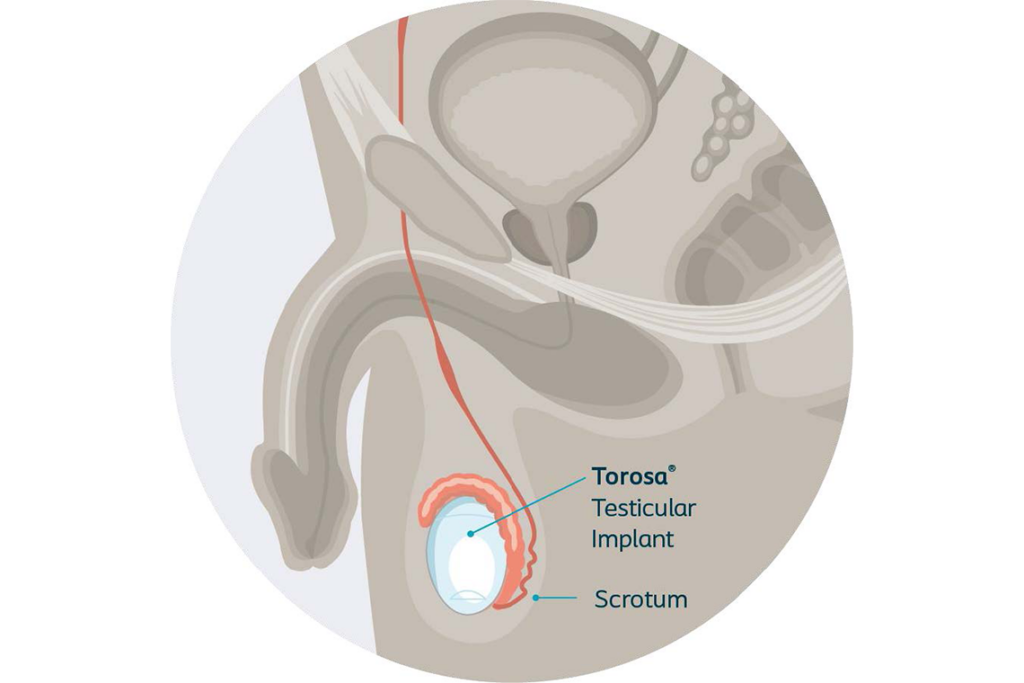
The Torosa implant is a saline-filled testicular implant that is placed completely inside the body, typically during an outpatient procedure. The Torosa implant aids in creating and restoring a more natural look and feel of the testicles in the scrotum.1
Rigorously tested and approved
The Torosa implant has been rigorously tested and is the only testicular implant with FDA approval.
88% of men implanted with a testicular implant reported that they would undergo the surgery again if it was necessary.2
Testicular implants are more than just a cosmetic solution
Testicular implants can help restore part of what was lost and have been shown to reduce the psychological impact resulting from loss or absence of a testicle.3

of men reported improved body image after receiving an implant4

Talk to your doctor about the Torosa implant
Following testicle removal, 98% of patients believe it’s important to be offered a testicular implant, but only 47% of them are.5,6
It’s not too early to ask if a testicular implant is right for you—and share your preferences around your treatment plan including whether the testicular implant can be placed at the same time as the testicle removal.

satisfaction with the Torosa implant1

would recommend to a friend2
Torosa implants are available in multiple sizes to help match the size of the testicle(s) that were removed.

Extra small

Small

Medium

Large
Frequently asked questions
Cost matters, but you have options
Testicular implants, including the Torosa implant, are prescribed by a physician. Cost and insurance coverage can vary based on many factors. Whether you’re fully covered or will be paying out of pocket, it’s important to understand your options.
Freeman, Torosa Implant Recipient
Learn more from someone who’s been there
Listen to a story of someone who has faced testicle removal and has experienced life with the Torosa testicular implant.
References
1 Data on file at Coloplast.
2 Clifford TG, Burg ML, Hu B, Loh-Doyle J, Hugen CM, Cai J, Djaladat H, Wayne K, Daneshmand S. Satisfaction With Testicular Prosthesis After Radical Orchiectomy. Urology. 2018 Apr;114:128-132.
3 Bodiwala D, Summerton DJ, Terry TR. Testicular prostheses: development and modern usage. Ann R Coll Surg Engl. 2007 May;89(4):349-53.
4 Hayon S, Michael J, Coward RM. The modern testicular prosthesis: patient selection and counseling, surgical technique, and outcomes. Asian J Androl. 2020 Jan-Feb;22(1):64-69.
5 Dieckmann KP, Anheuser P, Schmidt S, Soyka-Hundt B, Pichlmeier U, Schriefer P, Matthies C, Hartmann M, Ruf CG. Testicular prostheses in patients with testicular cancer – acceptance rate and patient satisfaction. BMC Urol. 2015 Mar 13;15:16.
6 Robinson R, Tait CD, Clarke NW, Ramani VA. Is it safe to insert a testicular prosthesis at the time of radical orchidectomy for testis cancer: an audit of 904 men undergoing radical orchidectomy. BJU Int. 2016 Feb;117(2):249-52.
PM-33605
Important safety information
Torosa® Saline-Filled Testicular Prostheses Important Safety Information
Torosa Saline-Filled Prosthesis is a surgically implanted artificial testicle designed to replicate the size, shape, and feel of one or two testicles following surgical removal, or the absence of a testicle (agenesis) in males.
Indications
The Coloplast Torosa Saline-Filled Testicular Prosthesis is intended for use when cosmetic testicular replacement is indicated i.e., in the case of agenesis or following the surgical removal of a testicle.
Contraindications
The implantation of testicular prosthetic implants is contraindicated in the presence of infection or abnormal growth (neoplasm).
Warnings
A testicular implant in patients with pre-existing enlargement of the scrotum (varicocele) may result in persistent pain. Due to the nature of silicone implants, testicular implants should not be considered lifetime implants and require replacement surgery over time. Torosa should typically not be implanted in patients with a documented sensitivity to silicone. These patients should discuss the risks of this implant with their physician. Patients with disorders such as lupus, scleroderma and myasthenia gravis should discuss the risks of this implant with their physician. Sepsis, hemorrhage or thrombosis may result from the placement of any foreign object in the body. An oversized implant can lead to potential complications such damage or loss of tissue (necrosis) or foreign body reaction leading to formation of blood clots (thrombosis). Excessive scarring and/or tightening around the implant (capsular formation or contracture) may occur.
Precautions
Discuss all available treatment options with your doctor to understand the risks and benefits of a testicular implant.
Potential Complications
The following device-related and procedure-related events were experienced during the clinical study for this device: discomfort/pain, swelling (edema), extrusion, displacement/migration, hematoma, abnormal tissue formation (keloid, fibrosis, granuloma), implant deflation, fluid accumulation (inguinal area), constipation, numbness/weakness (neuropathy) in legs/feet, and surgical site infection (abscess).
Advice to Patient
Resize of the implant may be elected in the future for young males implanted prior to body maturation.
This treatment is prescribed by your physician. Discuss the treatment options with your physician to understand the risks and benefits of the various options to determine if a testicular implant is right for you.
Caution: Federal law (USA) restricts this device to sale by or on the order of a physician.

