Testicular
replacement
Restore a more natural appearance
Losing one or both of your testicles isn’t an easy topic to discuss, but it can be a reality for many.
Men facing testicular replacement experience more than the physical effects of missing a testicle. The absence of a testicle has been shown to be a psychologically traumatic experience for males of all ages.1
32%
of testicular cancer survivors say they missed their testicles2
26%
of testicular cancer survivors have feelings of uneasiness or shame about their body2
43%
experience reduction
in sexual activity3

There are options available to help restore a part of what was lost
Even though testicular implants have been shown to reduce the psychological impact resulting from loss or absence of a testicle, less than half of patients are offered one when their testicle is removed.4
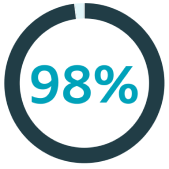
of orchiectomy (procedure to remove one or both testicles) patients believe it’s important to be offered a testicular implant5
But only

of patients are offered a testicular implant5
What causes a missing testicle?
There are many reasons why a testicle may need to be removed, including testicular cancer, testicular trauma and testicular torsion. Other causes of a missing testicle include undescended testicles and born without a testicle.1
If you have had or expect to have one or both of your testicles removed, there is hope.
Regardless of age, the loss of a testicle not only has impacts on physical appearance but can also impact your relationships and your self-esteem. The fact is, there is an alternative that can help restore part of what was lost.
References
1 Bodiwala D, Summerton DJ, Terry TR. Testicular prostheses: development and modern usage. Ann R Coll Surg Engl. 2007 May;89(4):349-53.
2 Skoogh J, Steineck G, Cavallin-Ståhl E, Wilderäng U, Håkansson UK, Johansson B, Stierner U; SWENOTECA. Feelings of loss and uneasiness or shame after removal of a testicle by orchidectomy: a population-based long-term follow-up of testicular cancer survivors. Int J Androl. 2011 Apr;34(2):183-92.
3 Rossen P, Pedersen AF, Zachariae R, von der Maase H. Sexuality and body image in long-term survivors of testicular cancer. Eur J Cancer. 2012 Mar;48(4):571-8.
4 Hayon S, Michael J, Coward RM. The modern testicular prosthesis: patient selection and counseling, surgical technique, and outcomes. Asian J Androl. 2020 Jan-Feb;22(1):64-69.
5 Dieckmann KP, Anheuser P, Schmidt S, Soyka-Hundt B, Pichlmeier U, Schriefer P, Matthies C, Hartmann M, Ruf CG. Testicular prostheses in patients with testicular cancer – acceptance rate and patient satisfaction. BMC Urol. 2015 Mar 13;15:16.
6 Robinson R, Tait CD, Clarke NW, Ramani VA. Is it safe to insert a testicular prosthesis at the time of radical orchidectomy for testis cancer: an audit of 904 men undergoing radical orchidectomy. BJU Int. 2016 Feb;117(2):249-52.
PM-33598





