Reclaim your confidence
with lasting, effective solutions for men’s intimate healthcare needs.

are affected by erectile dysfunction (ED) in the United States1
1 in 10 men

over the age of 60 experience urinary incontinence2
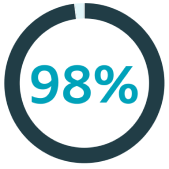
of orchiectomy patients believe it’s important to be offered a testicular implant3
Patients share their stories

Colin
Titan® Penile Implant Recipient
“What I appreciate most is not having to worry about ED getting in the way of our relationship.”
Colin’s story
ED Explained
The struggle with erectile dysfunction (ED) can affect self-confidence, put a strain on intimate relationships, and impact everyday life. This 11-part video series addresses questions, explores treatment options, and offers physician insights to guide you in your journey toward finding a solution.
Find a specialist
Explore our physician directory to find a local qualified urologist who specializes in men’s health. They can explain your treatment options, answer your questions, and help you take the next step toward restoring your health, and your confidence.
References
1 Burnett AL; Nehra A; Breau RH, et al. Erectile Dysfunction: AUA Guideline. J Urol. Sep 2018;200(3):633-641.
2 Shamliyan TA, Wyman JF, Ping R, Wilt TJ, Kane RL. Male urinary incontinence: prevalence, risk factors, and preventive interventions. Rev Urol. 2009;11(3):145-165.
3 Dieckmann KP, Anheuser P, Schmidt S, Soyka-Hundt B, Pichlmeier U, Schriefer P, Matthies C, Hartmann M, Ruf CG. Testicular prostheses in patients with testicular cancer – acceptance rate and patient satisfaction. BMC Urol. 2015 Mar 13;15:16.
PM-32538
Important safety information
Titan® & Titan Touch Inflatable Penile Prosthesis
The Titan Inflatable Penile Prosthesis is a surgically implanted mechanical penile implant intended for the treatment of erectile dysfunction in men. The Titan implant is a 3-piece fluid-filled system manually operated to produce and sustain an erection for sexual intercourse.
Indications
The Titan Inflatable Penile Prosthesis is indicated for male patients with erectile dysfunction who are considered to be candidates for implantation of a penile prosthesis.
Contraindications
The Titan implant is not for use in patients who have one or more of the following conditions: 1) have an active infection, particularly urinary tract or genital infection, 2) are sensitive or allergic to silicone or polyurethane, 3) have ongoing difficulty urinating or emptying the bladder (e.g., bladder outlet obstruction or neurogenic bladder), or 4) unwilling to undergo any further surgery for device revision.
Warnings
Patients should consider the warnings, precautions and potential complications associated with the use of this product, which may include the following: potential for resurgery (note: device is not a lifetime implant). Implantation makes latent natural erections, as well as other interventional treatment options, impossible. Implantation may result in penile shortening, curvature or scarring. Pre-existing abdominal or penile scarring or contracture may make surgical implantation more complicated or impractical. Diabetic, as well as immunocompromised patients, may have an increased risk of infection which could result in permanent damage to tissue/organs. Excessive stresses from rigorous exercise and vigorous masturbation/intercourse could lead to device damage. Certain stresses and pressures (straddle seating, obesity, etc.) could lead to involuntary inflation or deflation. Post-implant penile size, girth and angle can vary based on patient anatomy, implant size, level of inflation, and presence of Peyronie’s disease.
Precautions
Patients with spinal cord injury may have an increased risk of infection. This device may be used to treat erectile dysfunction in the presence of Peyronie’s disease. Although the implant is not visible, depending on the placement (submuscular) the reservoir may be palpable.
Patients should consider the following factors which could lead to increased risk of failure and can be critical to the eventual success of the procedure: ability and willingness of the patient to follow instructions; associated psychological status (e.g., psychogenic erectile dysfunction, inappropriate attitude or motivation); health conditions which hamper sexual activity (such as severe angina) may prevent successful use of this device; manual dexterity problems; and lack sufficient manual dexterity or strength necessary to operate the device.
Impact injuries to the pelvic or abdominal areas (e.g., sports injuries) can result in damage to the implant which may necessitate replacement of the device. Contracture of tissue around the pump can cause unnatural firmness in the scrotum and involuntary inflation or deflation. The device may fail to deflate and/or deflation of the device may be slow or difficult for some patients. Device malfunctions may result in the inability to inflate or deflate the device. Removal of the device without timely reimplantation of a new implant may complicate subsequent reimplantation.
Potential Complications
Adverse events are known to occur with penile protheses procedures and implants; some may require revision surgery or removal of the implant. Adverse events following penile protheses implantation may be new onset (de novo), persistent, worsening, transient, or permanent.
Adverse events may include but are not limited to: inability to pull foreskin back from tip of uncircumcised penis (acquired phimosis); abnormal wound healing/adhesion/scar tissue; bladder storage symptoms/urinary retention; tightening, shortening, deformity or curvature of penis (capsular contracture, induration); discomfort/pain; injury to tissue or organs (perforation/erosion/extrusion) resulting in damage or loss of tissue (necrosis); open tunnel between tissue or organs (fistula); foreign body reaction/allergic reaction/sensitivity; bleeding/hemorrhage or collection of blood or fluid outside of tissue or vessels (hematoma/seroma); hernia; Infection/urinary tract infection; redness or swelling (inflammation/edema); difficult or painful intercourse (dyspareunia/sexual dysfunction); obstruction/occlusion; numbness or decreased sensation (e.g., hypoesthesia); and urinary incontinence. The occurrence of these events may require one or more subsequent surgeries which may or may not always fully correct the complication.
This treatment is prescribed by your physician. Discuss the treatment options with your physician to understand the risks and benefits of the various options to determine if an inflatable penile implant is right for you.
Caution: Federal law (USA) restricts this device to sale by or on the order of a physician.
PM-15451 / Feb 2024
Genesis® Malleable Penile Prosthesis Important Safety Information
Genesis Malleable Penile Prosthesis is a surgically implanted penile implant intended for the management of erectile dysfunction (impotence) in men. The implant has two firm yet flexible silicone rods implanted in the penis to produce an erection for sexual intercourse. The device is manually positioned to simulate an erect or flaccid penis.
Indications
The implant is designed for the management of erectile dysfunction (impotence) stemming from a variety of causes, including: epispadias (a rare birth defect located at the opening of the urethra), pelvic fracture; spinal cord injury or disease; prostatectomy; cystectomy (surgical removal of the bladder); abdominal-perineal resection (surgical removal of the anus, rectum, and sigmoid colon); multiple sclerosis; diabetes mellitus; alcoholism; arteriosclerosis and hypertensive vascular disease; priapism (prolonged and painful erection of the penis); and Peyronie’s disease (curvature of the penis). The implant may also be used in selected patients with psychogenic impotence.
Contraindications
Implantation procedures are not advisable if infection is present anywhere in the body, especially urinary tract or genital infection. The implant should not be used in patients who have unresolved problems such as elevated residual urine from bladder outlet obstruction, or neurogenic bladder. The implant should be used with caution in diabetic patients who are more susceptible to infection and the complications of infection than nondiabetic patients. Other contraindications include unresolved urinary problems, any condition which may hamper sexual activity (such as severe angina), a history of sensitivity to foreign materials, compromised wound healing, compromised immune system, any anatomic or physiologic abnormality that could lead to significant postoperative complications, an unwillingness to undergo any further surgery for revision and psychological instability of the patient.
Warnings
Your doctor will advise you of all potential risks and complications associated with the proposed surgical procedure and device, including providing a comparison of the risks and complications of alternative procedures and implants. As a mechanical device, the implant can malfunction, wear out or be subject to misuse and may require replacement. Penile implants should not be considered lifetime implants. The implant should be used with caution in patients with borderline bladder decompensation or enlarged prostate. Patients, including paraplegics, should be free of indwelling catheters prior to implantation.
Precautions
Your doctor may conduct a thorough diagnostic evaluation, including psychiatric/sexual counseling. Your partner is encouraged to join the consultation.
Potential Complications
Complications may include, but are not limited to, the following: foreign body response; erosion; perforation; extrusion; infection; loss of tissue (necrosis); device malfunction (e.g., loss of rigidity, twisting, fracture, separation); impaired blood flow to penis; swelling (lymphedema) of the penis; hematoma; scarring; pain; incorrect implant position; deformity at the head of the penis; incorrect sizing; inability to pull the foreskin back from the tip of an uncircumcised penis (paraphimosis); voiding difficulty; decreased sensation and inflammation/irritation.
The implant may differ from original erection (e.g. not of equal length or girth) compared to what was previously experienced with natural erections.
This treatment is prescribed by your physician. Discuss the treatment options with your physician to understand the risks and benefits of the various options to determine if a malleable penile implant is right for you.
Caution: Federal law (USA) restricts this device to sale by or on the order of a physician.
PM-16813 / Apr 2024
Virtue® Male Sling System Important Safety Information
Virtue Male Sling is a polypropylene mesh device or “sling” intended to prevent involuntary urine leakage (incontinence) at times of increased pressure on the bladder (e.g., coughing, sneezing, laughing, lifting heavy objects, exercise). It is permanently implanted to support, elevate and gently compress the urethra, the tube that connects to the bladder to carry urine outside of the body.
Indications
The Virtue Male Sling System is an implantable, suburethral support sling indicated for the treatment of male stress urinary incontinence (SUI).
Contraindications
The Virtue Male Sling is contraindicated in patients with one or more of the following conditions: 1) documented hypersensitivity or allergic reaction to polypropylene, 2) active infection, including untreated urinary tract and/or infection in the operative field, 3) patients with untreated or serious blood clotting (coagulation) disorders, 4) patients with blockage of urine flow (obstructive uropathy), 5) patients under the age of 18.
Warnings
Your physician will conduct a complete evaluation with testing to confirm you are a candidate for a male sling. Patients will then be advised prior to surgery, of the warnings associated with the use of this product, the associated surgical and postoperative risks and potential complications. Sling associated complications may result in resurgery which may lead to partial or complete removal of the sling. Complete removal of the sling may not always be possible, and removal may not fully correct these complications. New onset (de novo) complications may occur. As with all surgical procedures, patients with certain underlying conditions may be more susceptible to postoperative bleeding, impaired blood supply, compromised/delayed healing, sling exposure or other complications and adverse events. The risk versus benefit of the male sling should be considered in patients with one or more of the following conditions: auto-immune disease, blood clotting (coagulation) disorder, connective tissue disease, impaired immune system (debilitated or immunocompromised state), diabetes, pelvic radiation therapy, physical characteristics (e.g., body mass index), kidney problems (renal insufficiency), smoking related conditions (e.g., COPD, chronic cough).
Potential Complications
Adverse events are known to occur with sling procedures and implants. Adverse events following sling implantation may be immediate or delayed, localized or systemic, new onset (de novo) or worsening, acute or chronic, transient or permanent.
Adverse events may include but are not limited to: allergic reaction, hypersensitivity; abnormal immune response (autoinflammatory/autoimmunity syndrome); bladder symptoms (e.g., increased daytime frequency, urgency, nocturia (urinating more than once per night), overactive bladder, urinary incontinence); bleeding/hemorrhage; delayed/impaired/abnormal wound healing; exposure, extrusion or erosion of sling into other structures or organs; fistula formation (abnormal connection or passageway between two structures in the body); foreign body granuloma (abnormal tissue formation)/scar tissue formation; genital burning / tingling / numbness (paresthesia); infection; tissue swelling/redness/discomfort (inflammation/irritation); avoidance/difficult/painful intercourse (dyspareunia / sexual dysfunction); tissue death (necrosis); weakness or loss of sensation (neuromuscular disorder); palpable mesh; pain; perforation or injury to adjacent muscles, nerves, vessels, structures, or organs (e.g., bone, bladder, urethra, ureters, bowel); collection of clear fluid or blood outside of tissue or vessels (seroma/hematoma); sling migration; urinary tract infection; inability to completely empty bladder (urinary tract obstruction); voiding symptoms (e.g., dysuria (painful urination), urinary retention, incomplete emptying, bladder outlet obstruction, straining, position-dependent voiding, slow stream).
This treatment is prescribed by your physician. Discuss the treatment options with your physician to understand the risks and benefits of the various options to determine if a male sling is right for you.
Caution: Federal law (USA) restricts this device to sale by or on the order of a physician.
PM-15544 / Apr 2024
Torosa® Saline-Filled Testicular Prostheses Important Safety Information
Torosa Saline-Filled Prosthesis is a surgically implanted artificial testicle designed to replicate the size, shape, and feel of one or two testicles following surgical removal, or the absence of a testicle (agenesis) in males.
Indications
The Coloplast Torosa Saline-Filled Testicular Prosthesis is intended for use when cosmetic testicular replacement is indicated i.e., in the case of agenesis or following the surgical removal of a testicle.
Contraindications
The implantation of testicular prosthetic implants is contraindicated in the presence of infection or abnormal growth (neoplasm).
Warnings
A testicular implant in patients with pre-existing enlargement of the scrotum (varicocele) may result in persistent pain. Due to the nature of silicone implants, testicular implants should not be considered lifetime implants and require replacement surgery over time. Torosa should typically not be implanted in patients with a documented sensitivity to silicone. These patients should discuss the risks of this implant with their physician. Patients with disorders such as lupus, scleroderma and myasthenia gravis should discuss the risks of this implant with their physician. Sepsis, hemorrhage or thrombosis may result from the placement of any foreign object in the body. An oversized implant can lead to potential complications such damage or loss of tissue (necrosis) or foreign body reaction leading to formation of blood clots (thrombosis). Excessive scarring and/or tightening around the implant (capsular formation or contracture) may occur.
Precautions
Discuss all available treatment options with your doctor to understand the risks and benefits of a testicular implant.
Potential Complications
The following device-related and procedure-related events were experienced during the clinical study for this device: discomfort/pain, swelling (edema), extrusion, displacement/migration, hematoma, abnormal tissue formation (keloid, fibrosis, granuloma), implant deflation, fluid accumulation (inguinal area), constipation, numbness/weakness (neuropathy) in legs/feet, and surgical site infection (abscess).
Advice to Patient
Resize of the implant may be elected in the future for young males implanted prior to body maturation.
This treatment is prescribed by your physician. Discuss the treatment options with your physician to understand the risks and benefits of the various options to determine if a testicular implant is right for you.
Caution: Federal law (USA) restricts this device to sale by or on the order of a physician.



